Abstract
Background
Relapse free survival of pediatric AML remains only 60%. Current standard myelosuppressive therapy has been maximized, so novel therapies with minimal toxicities are needed to improve outcomes. Previously, we found atovaquone (AQ), an FDA-approved drug that treats pneumocystis jiroveci pneumonia (PJP), reduces AML burden - by suppressing oxidative phosphorylation (OXPHOS) - in xenograft mice. Clinically achievable concentrations of AQ for anti-PJP are 40-80µM, but the anti-leukemia effects are observed as low as 10µM (Stevens et al, Bld Adv, 2019). This makes AQ an ideal drug to incorporate into AML treatment. AQ is a daily administered oral medication, and plasma levels depend on patient compliance, absorption, and entero-hepatic recirculation, which can be compromised due to the patient population and adverse events (AE) of chemotherapy. Here we investigated the feasibility of incorporating AQ into standard pediatric AML treatment.
Methods
Patients with de novo AML were enrolled at two children's hospitals in the USA. Daily administration of AQ at established PJP prophylaxis dosing was combined with standard chemotherapy for AML, based on the Medical Research Council (MRC) backbone of cytarabine 100mg/m2 q12h x 10 days, and daunorubicin 50mg/m2/dose on days 1, 3, and 5. As it was unclear if our AQ dosing would provide adequate PJP prophylaxis, it was left to provider discretion to give additional PJP protection. AQ compliance, AEs (per NCI CTCAE v5), parent/caregiver ease of administration score (scale: 1-5, 1=very difficult, 5=very easy to administer) and peripheral blood/bone marrow pharmacokinetics (PK) were collected during Induction 1. Real time AQ plasma concentration results were not provided. To address feasibility of achieving adequate levels, all gastrointestinal (GI) AEs ≥ grade 2 were collected, in addition to grade 4 or greater AEs. Patients who took at least 85% of planned doses and missed less than 2 consecutive doses of AQ were eligible for analyses. Correlative biology studies assessed AQ induced apoptosis at 30uM, effects on OXPHOS and relevant signaling activities. Patient derived xenografts (PDX) were established and treated with AQ. This trial is registered with ClinicalTrials.gov (NCT03568994).
Results
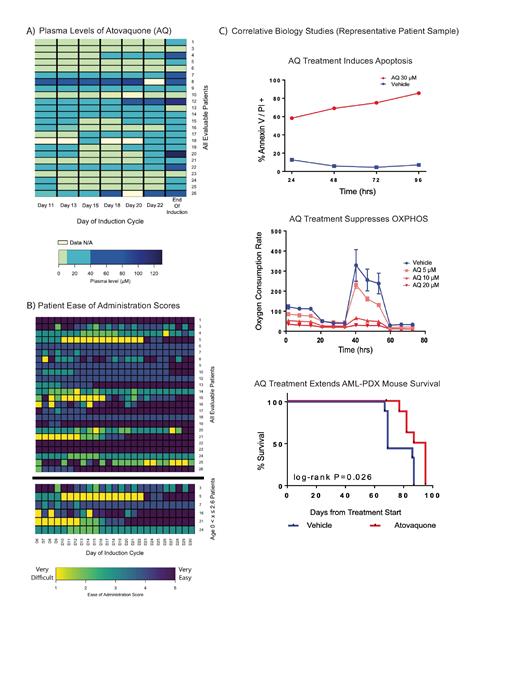
A total of 26 pediatric AML patients enrolled (ages 8 months - 19.7 years, mean 10.7 years); 24 patients were evaluable for this study. Two patients had Grade ≥ 3 GI toxicities that prohibited enteral administration so they were excluded from AQ PK and ease of administration analyses. We found that 14/24 (58%) patients achieved plasma levels above the target anti-leukemia concentration (10µM) by day 11. At the end of induction, 19/24 (75%) patients achieved plasma levels above 10µM, but only 7/24 (29%) patients achieved adequate levels for PJP prophylaxis (40µM). Only 1 patient achieved levels above 40µM throughout the trial [FIG A]. Mean ease of administration score was 3.8. For the youngest patients (x ≤ 2.6years), the average score was 3.4 which was not significantly different from older patients (ANOVA, p > 0.05) [FIG B]. Ease of administration scores showed no association with plasma levels (Pearson's correlation, p > 0.05). Finally, correlative biology studies in patient samples demonstrated robust AQ-induced apoptosis, OXPHOS suppression, and prolonged survival in a PDX model receiving AQ [FIG C].
Conclusion
Our data demonstrate the feasibility of combining AQ with traditional chemotherapy for pediatric AML. Patients of all ages were able to tolerate AQ and no AEs were attributable to AQ administration. The target anti-leukemic concentration of AQ in the plasma (> 10uM) was frequently achieved, but concentrations of > 40uM at standard dosing were rare. Low plasma levels of AQ did not correlate with the presence of GI related AEs or weight loss, so plasma levels should be monitored to ensure sufficient PJP prophylaxis. Our correlative biology results support suppression of OXPHOS as the primary mechanism of action by which AQ exerts its anti-leukemia effect, and AQ may be an active anti-leukemia agent for pediatric AML patients.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal